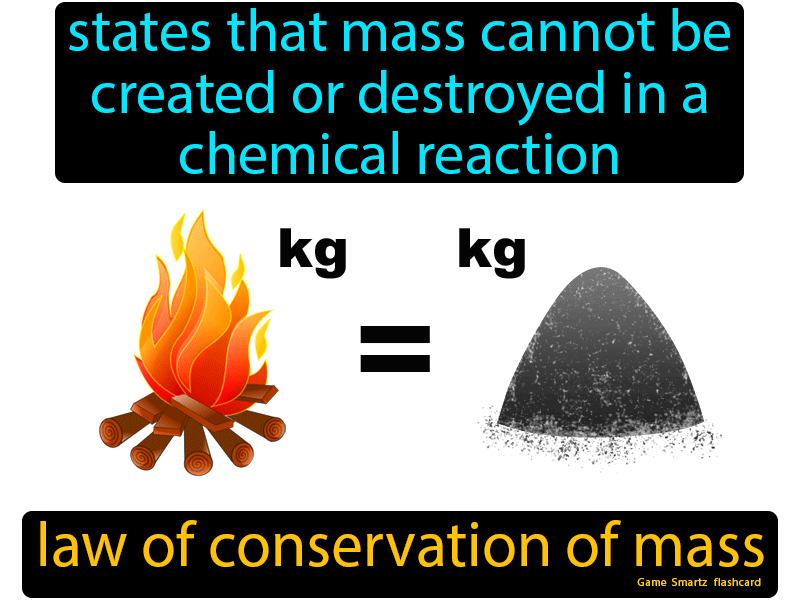
law of conservation of mass
The conservation of energy is a fundamental concept of physics along with the conservation of mass and the conservation of momentum. Two bodies of mass M and m are moving in opposite directions with the velocities v.

Law Of Conservation Of Mass Infographic Diagram Showing A Lab Experiment Between Reactants And Product Conservation Of Mass Science Education Chemistry Lessons
One common example youll come across is the image of a bonfire or campfire.

. This means the total mass of reactants in a chemical reaction will equal the total mass of the products. Where we have identified the physical meaning of the terms in the left and right sides of the equation. The mass can neither be created nor destroyed. The law of conservation of mass was created in 1789 by a French chemist Antoine Lavoisier.
At the beginning of the 20th century the notion of mass underwent a radical revision. One of the striking results of Einsteins theory of relativity is that mass and energy are equivalent and convertible one into the other. The Law of Conservation of Matter Conservation of Mass. Instead the atoms join together in different ways to form products.
The total mass stays the same during a chemical reaction. The law of conservation of mass is useful for a number of calculations and can be used to solve for unknown masses such the amount of gas consumed or produced during a reaction. The law of conservation of energy states that energy can neither be created nor destroyed - only converted from one form of energy to another. Law of Conservation of Mass-Energy Mass-Energy Equivalence.
The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. M in the control volume. This is particularly confusing in the case of non-conservative forces where energy is converted from mechanical energy into thermal. Youve gathered some sticks with friends and lit them with a.
Now we can rewrite the principle of conservation of mass given in Equation 1 as CS dM dA dt ρ V n 7 Rate of Rate of net inflow of mass. The law of conservation of mass states that in a chemical reaction mass is neither created nor destroyed. History credits multiple scientists with discovering the law of conservation of mass. The law of conservation of mass indicates that mass cannot be created nor destroyed.
Similarly the law of conservation of energy states that the amount of energy is neither created nor destroyed. Here are two examples to help illustrate how this law works. The mass can neither be created nor destroyed. The law of conservation of energy is a physical law that states energy cannot be created or destroyed but may be changed from one form to another.
Newtons laws of motion that the total momentum remains constant in a system completely separated from external factors. The law requires that during any. Russian scientist Mikhail Lomonosov noted it in his diary as a result of an experiment in 1756. Burning of wood is a conservation of mass as the burning of wood involves Oxygen Carbon dioxide water vapor and ashes.
Chemical equations that do not obey this regular law of conservation of mass are known as unbalanced equations or skeleton equations. In both examples the reactants and products. The law of conservation of energy states that the total energy of an isolated system remains constant. In a steady state situation the time rate of change of the.
The law of conservation of mass was challenged with the advent of special relativity. Mass lost its absoluteness. In one of the Annus Mirabilis papers of Albert Einstein in 1905 he suggested an equivalence between mass and energy. Exact conservation laws include conservation of mass and energy conservation of linear momentum conservation of angular momentum and conservation of electric chargeThere are also many approximate.
For example when wood burns the mass of the soot ashes and gases equals the original mass of the charcoal and the oxygen when it. In physics a conservation law states that a particular measurable property of an isolated physical system does not change as the system evolves over time. The law of conservation of momentum can be explained from the second law of motion. The law of conservation of mass states that no atoms are lost or made in a chemical reaction.
The law of conservation of mass is known by some as Lavoisiers Law. This is the law of conservation of mass. For example the carbon atom in coal becomes carbon dioxide when it is burned. The Law of Conservation of Matter Conservation of Mass.
Another way of stating this law of chemistry is to say the total energy of an isolated system remains constant or is conserved within a given frame of reference. Equivalence of the mass and energy is described by. Best Example to Understand the Law of Conservation of. Law of conservation of energy is a false idea.
ReactantAny of the participants present at the start of a chemical reaction. If a gas is produced during a reaction which mass is often forgotten when calculating the final mass because the students are unable to see the gas. Law of Conservation of Mass Examples Combustion process. According to the second law the momentum of each changes at a rate equal.
Conservation law in physics a principle that states that a certain physical property that is a measurable quantity does not change in the course of time within an isolated physical system. In 1774 French chemist Antoine Lavoisier meticulously documented experiments that proved the law. Ct 2018 35 Mass. Also a molecule before it undergoes a chemical change.
Thus the amount of matter cannot change. This theory implied several assertions like the idea that internal energy of a system could contribute to the mass of the whole system or that mass could be converted into. The law requires that during any nuclear reaction radioactive. The law of conservation of mass is observed either in a balanced chemical equation which is a formulation that shows all mass is conserved throughout the reaction process.
Law of conservation of mass examples are useful for visualizing and understanding this crucial scientific concept. In classical physics such laws govern energy momentum angular. Areas of focus include the best definition of the law of conservation of mass and the weights of products in a given reaction using the law of conservation of mass. To get one molecule of H 2 O water with the molecular weight of 10 Hydrogen with molecular weight 2 is added with Oxygen whose molecular weight is 8 thereby.
Sir Antoine Lavoisier promoted this idea. The law of conservation of matter or principle of matter conservation states that the mass of an object or collection of objects never changes over time no matter how the constituent parts rearrange themselves. Since there is no external force acting on the system of two bodies momentum will be conserved. Web sources Handbook on fence viewers and laws on fences in the.
This law says that when a chemical reaction rearranges atoms into a new product the mass of the reactants chemicals before the chemical reaction is the same as the mass of the. For this reason balloons or zip. The law of conservation of matter or principle of matter conservation states that the mass of an object or collection of objects never changes over time no matter how the constituent parts rearrange themselves. Energy can be converted from one form to another potential energy can be converted to kinetic energy but.
Law Reporter 113 2018 A conservation council as grantee of the conservation restriction on defendants property may not recover monetary damages from defendants because defendants cut trees on their property in violation of the conservation restriction. The law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction. Law of conservation of massA law that states that mass. Within some problem domain the amount of energy remains constant and energy is neither created nor destroyed.
Law of Conservation of Mass. If they collide and move together after the collision we have to find the velocity of the system. This means that a system always has the same amount of energy unless its added from the outside. Increase of into the control volume.
The carbon atom changes from a solid structure to a gas but its mass does not change.

Law Of Conservation Of Mass Vector Illustration Labeled Educational Scheme With Substance Example Exp Conservation Of Mass Science For Kids Chemistry Lessons

How I Teach The Law Of Conservation Of Mass Conservation Of Mass Science Lessons Middle School Chemistry

Law Of Conservation Of Mass Cbse 9 Youtube Conservation Of Mass How To Memorize Things Chemical And Physical Changes
Law Of Conservation Of Mass Easy Science Conservation Of Mass Mass Activities Teaching Matter

What Is The Law Of Conservation Of Mass Chemistry For All Fuseschool Conservation Of Mass Chemistry Chemistry Education
Posting Komentar untuk "law of conservation of mass"